DIFFER researchers have constructed an ingenious plasma-activated electrolysis device that effectively breaks nitrogen gas and splits water into the energy-rich products hydrogen and nitric oxide. Water reduction and nitrogen fixation are key processes in providing fuels and chemical feedstock for wide variety of societal purposes. The sustainable challenge lies in designing all-electric sustainable pathways to replace the conventional, high-temperature industrial processes needed to produce these molecules. The DIFFER device integrates both in an elegant way and recently published their results in ACS Energy Letters!
Plasma-activated electrolysis
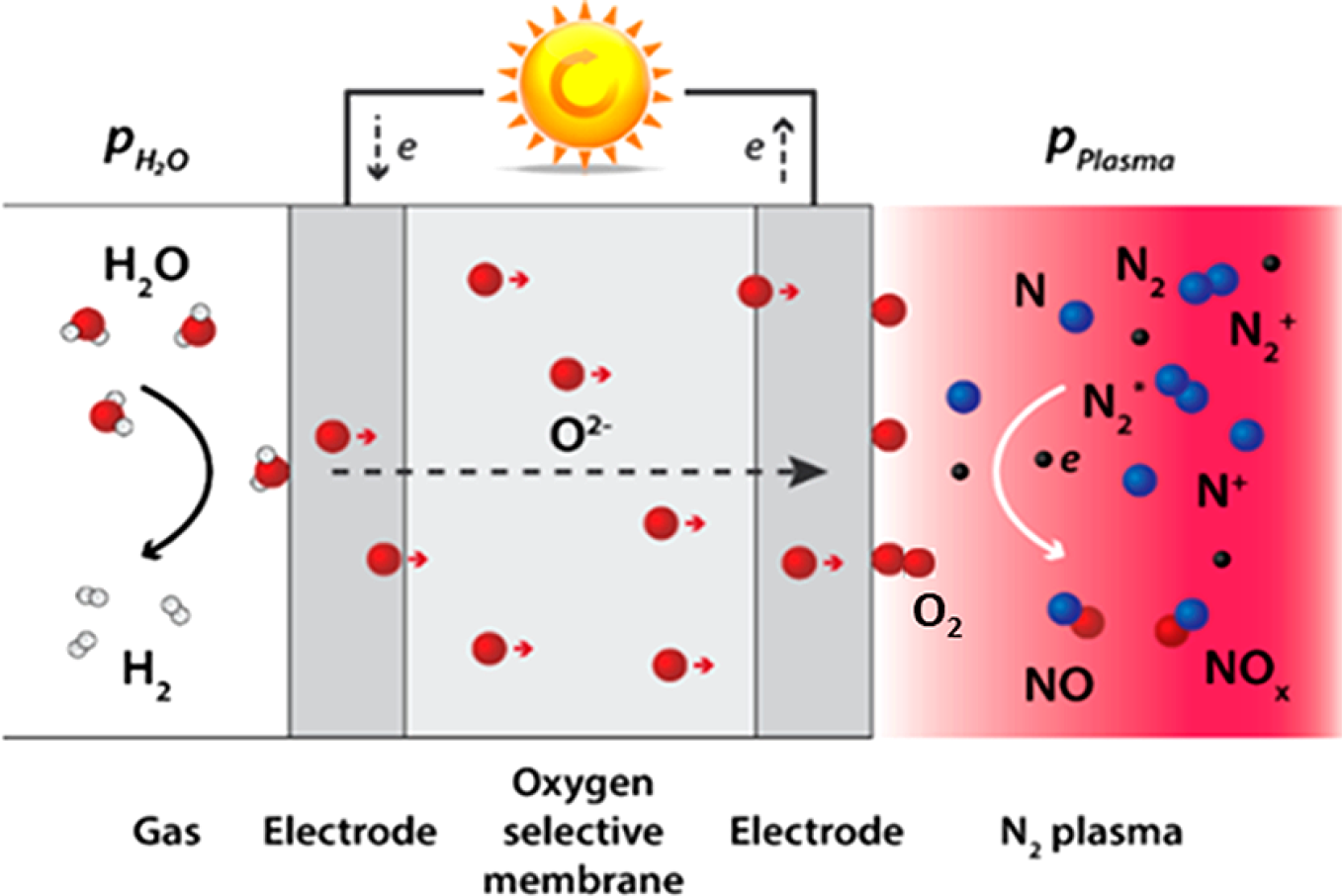
Group leader Mihalis Tsampas explains: “What is groundbreaking in this approach is that we manage to simultaneously and separately produce H2 from water and NO from nitrogen and oxygen in a single full-electric device. Key is that we use plasma-activation in the setup to boost the nitrogen chemistry at the second electrode to yield a NO concentration over 1000 times greater than for typical thermodynamic processes at a temperature of 650 °C." “This temperature is needed for efficient operation of solid-oxide electrolyzers to split water at the electrode and have subsequently the oxygen ions transported through the membrane,” adds post-doc Rakesh Sharma, “but realize it is very low compared the 2000 °C typical for industrial conditions when thermally oxidizing nitrogen.”
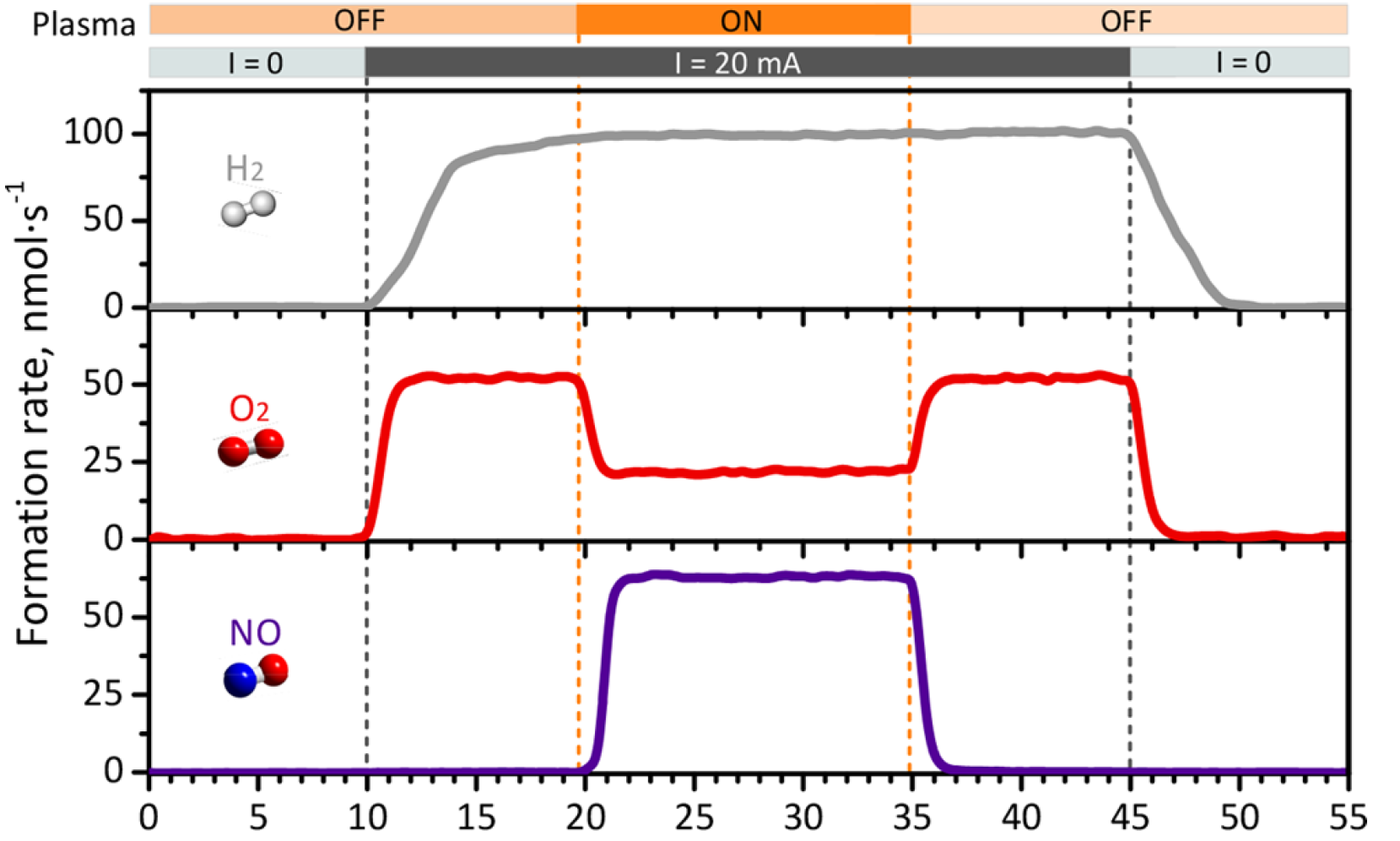
The plasma basically serves as a means to activate the nitrogen gas molecules, just before they encounter the oxygen molecules. The controlled plasma conditions are such that the nitrogen bonds are energized but without breaking the molecule. Post-doc researcher Hrishikesh Patel working on the project and first author of the paper adds: “By allowing these activated nitrogen molecules to react with the oxygen from the water splitting, we effectively demonstrated nitrogen fixation into NO at a low temperature, which lies into realm of all-electric processes.”
Our results clearly demonstrate the power of adding a plasma to state-of-the-art electrolysis. Future research will focus on unravelling the key species and the involved reaction mechanisms to truly understand and exploit the full potential.
Publication
Hrishikesh Patel, Rakesh Sharma, Vasileios Kyriakou, Arunkumar Pandiyan,Stefan Welzel, Richard van de Sanden, and Mihalis Tsampas, Plasma-activated electrolysis for cogeneration of nitric oxide and hydrogen from water and nitrogen, ACS Energy Letters (2019).
Go to the News page.