The key to boosting hydrogen storage in metal nanoparticles is understanding the role of defects, conclude physicist Andrea Baldi (Dutch Institute for Fundamental Energy Research DIFFER) and scientists from Delft, Amsterdam and Groningen in Physical Review Letters. Their work resolves a long-standing debate on what are the major factors influencing hydrogen absorption in nanoscale metals, something that could lead to the storage of clean power in hydrogen fuel at industrial capacity.
Hydrogen storage in nanoscale metals
Metals like magnesium can absorb and store hydrogen gas in their structure, very much like sponges are capable of absorbing water. This phenomenon could be used to store and transport clean hydrogen fuel produced from intermittent solar or wind power. Researchers have shown that using nanostructured metals could bring the process closer to practical temperatures and pressures. Understanding the factors behind the enhanced performance of nanoscale materials, however, is still a challenge.
Increased hydrogen uptake - stress or alloy effect?
Already in 2009, scientists from Amsterdam and Delft discovered that they could boost the pressure at which magnesium absorbs hydrogen over 200 times, by shrinking their sample size to the nanoscale and encapsulating them into a rigid palladium scaffold. If they could predict how this effect worked, they would have a pathway to improved hydrogen storage. The group theorized that the added stress due to the presence of palladium explained the higher uptake pressure. Researchers from Stanford University, however, later countered that an alloying effect between magnesium and palladium was responsible. Without a definitive understanding, there was no clear way to further optimise the absorption process.
The surprise ingredient: structural changes
First author of the original Dutch research Andrea Baldi set out to solve the hydrogen dispute by analysing hydrogen uptake in samples of magnesium alloyed with different amounts of palladium and under a wide variety of temperatures and pressures.
Andrea Baldi: "It turns out that both previous groups were off the mark. We see both stress and alloying effects, but neither can fully explain what happens when hydrogen is absorbed and released in these nanoscale materials. A critical missing ingredient in both models is the influence of defects." These defects modify how nanoscale metals can expand and deform internally to accommodate more hydrogen in ways that are very different from macroscopic “bulk” samples.
Applications beyond hydrogen storage
Andrea Baldi thinks his finding can have an impact beyond hydrogen storage. "Many energy- and information-storage systems rely on structural or phase changes in nanoscale materials, for instance the lithium-ion battery in your mobile phone or the data storage hard drive in your laptop. The absorption of hydrogen in nanomaterials turns out to be an excellent model system to study many of these processes. Understanding how hydrogen is absorbed in nanoscale materials can therefore have far-reaching implications."
Publication
Elastic versus alloying effects in Mg-based hydride films, Physical Review Letters 121, 255503
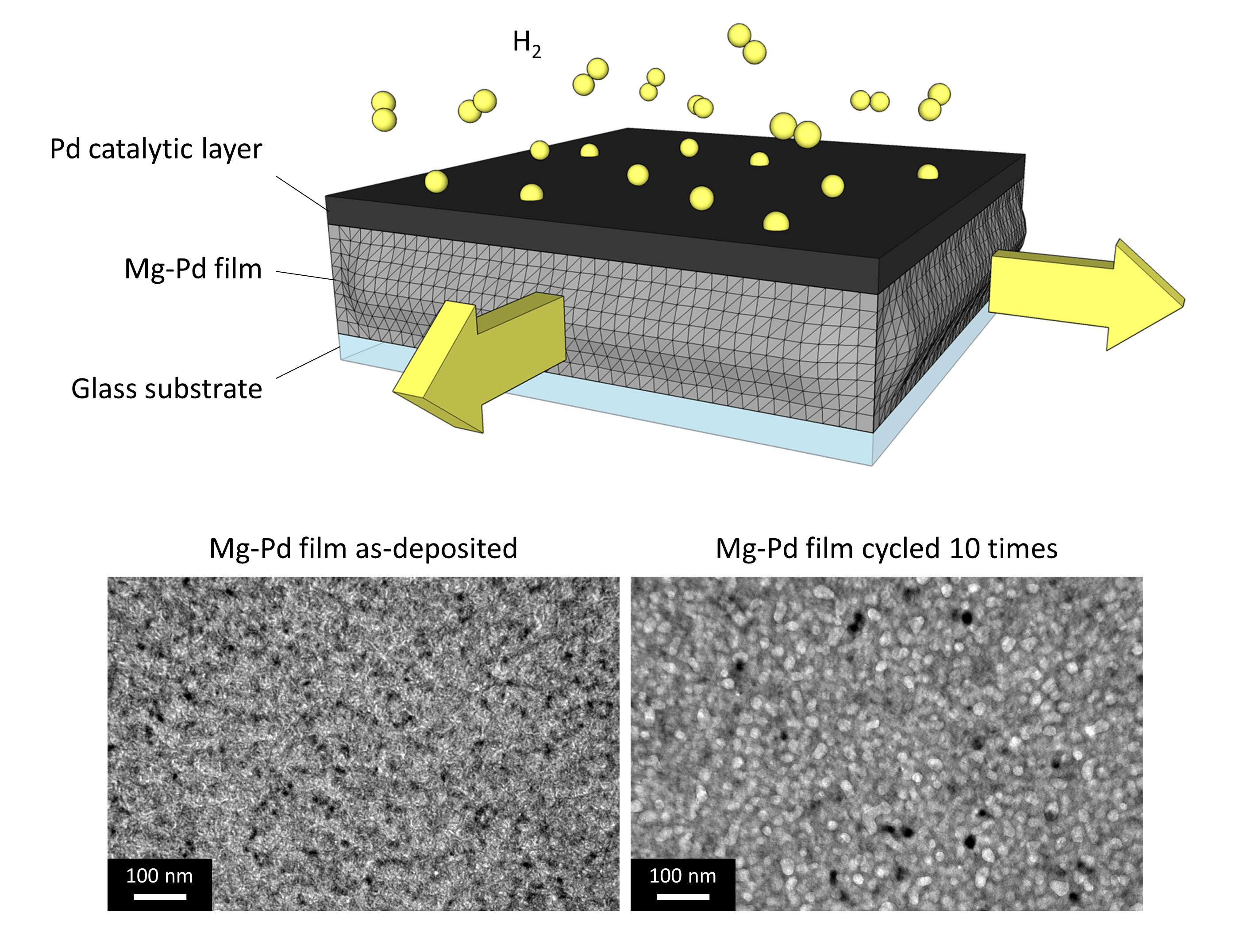
In these films, the adhesion between the top Pd layer and the Mg-Pd layer underneath counterbalances the expansion due to hydrogen absorption that, under normal circumstances, would tend to make the material expand by ~10% in all directions.
As a consequence of these "elastic constraints" the film can only expand vertically. After multiple cycles, it breaks into coarse grains and voids (the lighter areas in the bottom right image), as shown in the transmission electron microscopy images.
Go to the News page.