Scientists are looking for ways to make chemical reactions run on light. This may open the possibility to synthesize nanomaterials with unique properties and to steer chemical reactions towards new products. In one approach, metal nanoparticles added to a reactor can selectively absorb light and convert its energy to promote the growth of a thin shell of a different metal around them. PhD student Rifat Kamarudheen and his colleagues at DIFFER and Eindhoven University of Technology set out to discover exactly how this process works. The answer, which they describe in a recent article in the journal ACS Nano, surprised them.
Publication
Quantifying Photothermal and Hot Charge Carrier Effects in Plasmon-Driven Nanoparticle Syntheses
Rifat Kamarudheen, Gabriel W. Castellanos, Leon P. J. Kamp, Herman J. H. Clercx, and Andrea Baldi
ACS Nano, doi: 10.1021/acsnano.8b03929
"The basic idea is that we can add light as an ingredient in our chemistry and use it to activate and control the rate of different reactions", explains DIFFER's Andrea Baldi, who heads the Nanomaterials for Energy Applications group where the research was performed. "The tricky part is that light isn't just another powder you can add to the mix - you have to properly control how it enters the reaction mixture, how much of it is absorbed and how much is reflected out."
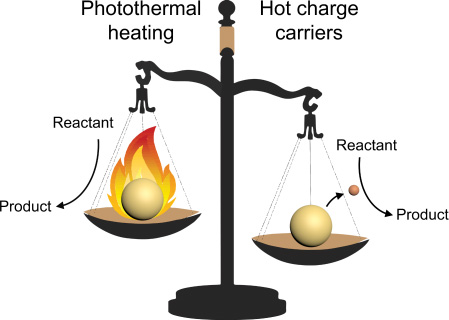
Heaters or electron sources?
The big question for the researchers was not if metal nanoparticles can use light to enhance chemical reactions - this effect has been described before. But how exactly was still very much an unsolved question. Is it just a case of photothermal heating of the surrounding medium by the nanoparticles, making chemical reactions run faster? Or is the rather exotic mechanism of injection of hot electrons at play, where the energy from the light is used to transfer electrons with high kinetic energy from the nanoparticles to chemicals at their surface?
Baldi: "I'll admit that I have always been pretty skeptical about hot electrons - to me the proof was never solid. So we designed our experiments to try to disprove their existence and demonstrate that all the observed reactivity was due to pure heating effects."
To tease apart possible reaction mechanisms, PhD student Rifat Kamarudheen mixed gold nanoparticles into a room temperature solution and tried to use light to grow a silver layer on top of them. One key ingredient to his experiment was the use of a chemical that strongly bonds to silver ions in solution and prevented the formation of metallic silver shells on top of the gold nanoparticles at room temperature. The formation of the silver shell, however, can be triggered either by heating the solution or by shining light on it.
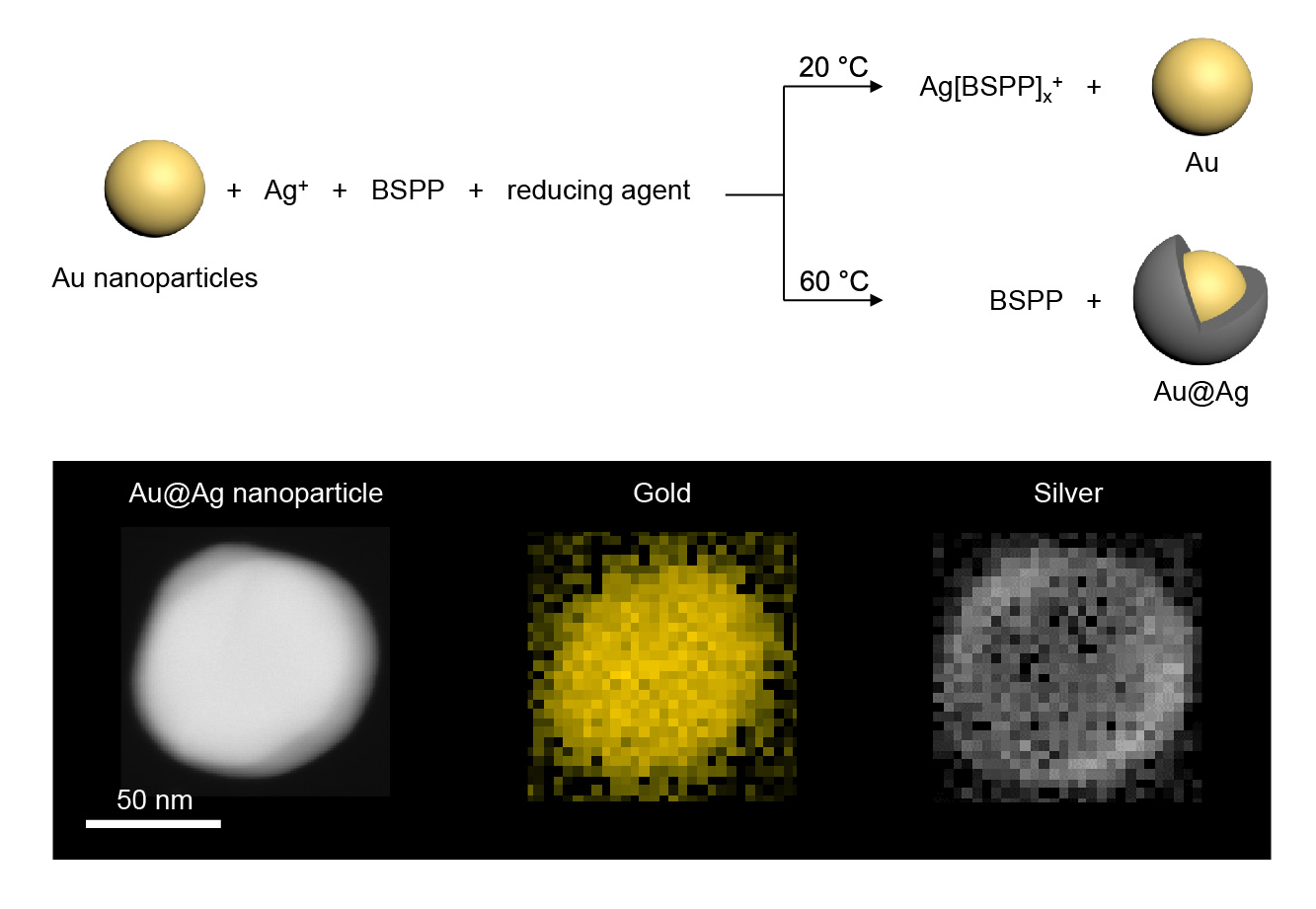
the reaction mixture: at room temperature no shell is formed, while at 60°C the reaction proceeds rapidly.
Equivalent temperature
Temperature is a crucial factor in chemistry: the higher the temperature, the more energy molecules and ions have and the easier they can overcome reaction activation barriers. Baldi and his team first proved that the silver shells did form when the solution was heated up. They then compared these results from conventional heating with the effect of activating the silver shell growth using a laser without any external heating. They found that under the effect of the laser alone, the silver shell was growing at a rate comparable to the one they observed for a solution heated to 50 °C in the dark. The question was, how much of this effect was photothermal? Were hot electrons involved at all?
Understanding the contribution of photothermal effects required a detail modeling of how light is absorbed and reflected (scattered) by the metal nanoparticles, as well as knowledge of the heat losses in the reactor. With the help of scientists from the Eindhoven University of Technology, Kamarudheen calculated that the irradiated gold nanoparticles were pumping enough heat into the solution to raise its temperature only to about 37°C. Because chemical reactivity rises sharply with temperature, such photothermal heating could only account for about 30% of the total observed reactivity under laser irradiation. The remaining 70%, conclude the researchers, cannot be attributed to classical thermal effects and has to come from hot electrons.
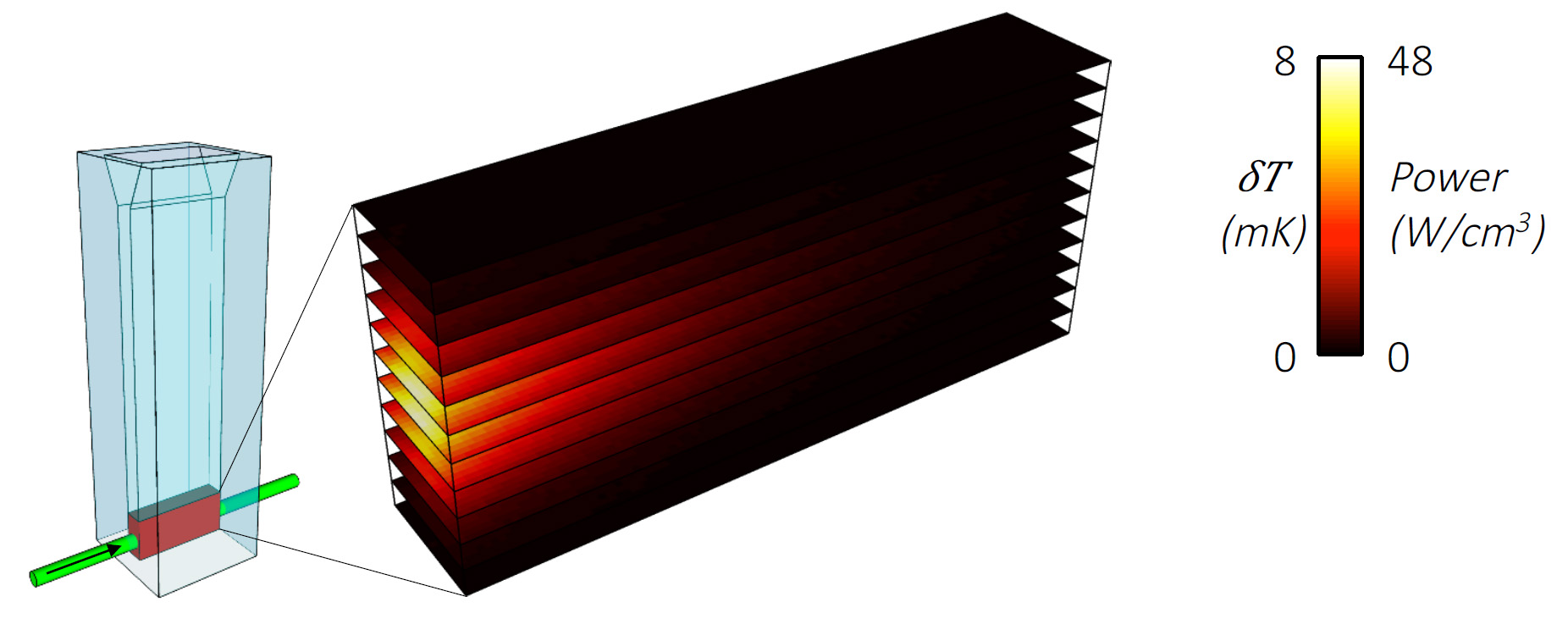
light-driven synthesis and where light is absorbed by the solution of gold nanoparticles.
Tuning for effect
"From the results it's clear that the bulk of the reactivity in our setup does not come from photothermal effects", says Baldi. "Despite our best effort to discredit their existence, it seems that hot electrons are still in the race!" The researchers have also shown that it is possible to tune the relative contribution of photothermal and hot electron effects, by changing the density of nanoparticles and the fraction of liquid that is irradiated by the laser. PhD student Rifat Kamarudheen: "These results are thrilling because they give us insights to selectively trigger photothermal or hot charge carrier effects, which can be beneficial in enhancing the efficiency of a wide variety of light-driven processes."
"Despite our results, in this paper we can still only present indirect evidence for the existence of these non-equilibrium electrons", says Baldi. Will he find a way to directly confirm their existence? "Let's just say that there's very exciting work in the pipeline..."
Go to the News page.