“It’s like watching atoms wrestle for oxygen.” DIFFER-researcher Vasileios Kyriakou describes the formation process of nanoparticles, seen in real-time with an advanced electron microscope. An international team of researchers observed the controlled formation of nanoparticles in inorganic perovskite materials. The result is published in ACS Nano and was highlighted by Science.
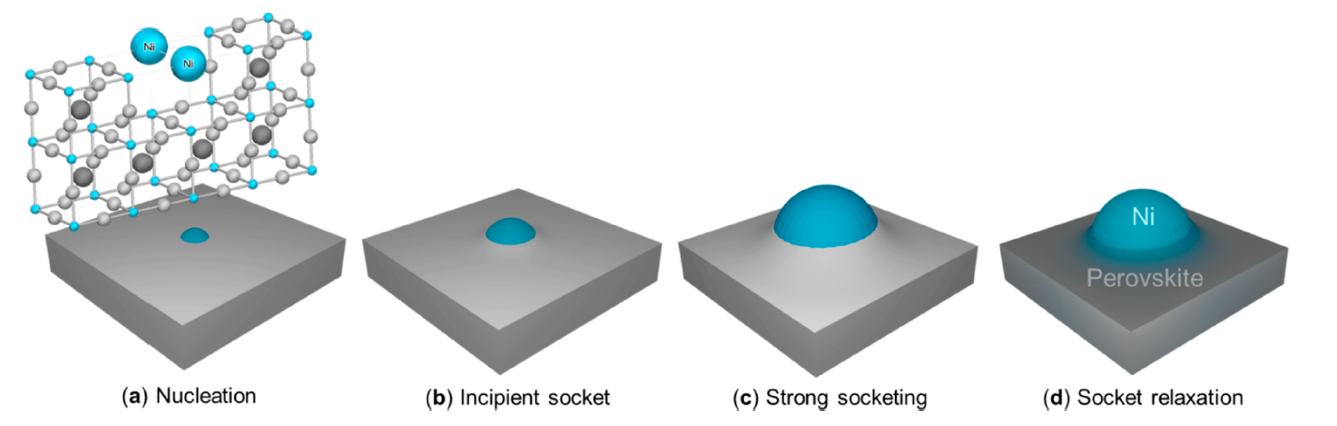
“It is almost like watching lanthanum, calcium, nickel and titanium atoms wrestle for too few oxygen atoms in their perovskite atomic lattice”, researcher Vasileios Kyriakou tells. “Seeing clusters of nickel atoms being pushed out in a very controlled manner, as they appear to be the weakest in holding on to the oxygen.” His colleague Mihalis Tsampas adds: “And the effect is that you end up with evenly distributed nickel nanoparticles, still partly embedded in the surface.”
Perovskite: new kid on the block
It’s science in action, or better to say: detailed understanding on how to fully tailor the process of exsolution to produce advanced energy materials. Perovskites are the new kid on the block for various energy application, ranging from oxygen transporting membranes in advanced electrolyzer concepts and catalytic convertors. Especially using them for catalytic and energy conversion schemes requires nanoparticles dispersed on their surface. That leads to two key questions critical for the performance of energy materials: how to get these nanoparticles evenly distributed over the perovskite surface? And if you manage to achieve that, how to anchor this particles properly to the surface?
Graphic: Schematic of exsolution process of Nickel from the perovskite oxide structure when exposing the surface to reducing conditions, in short “experimentally observed + precisely modelled -> fully understood -> tailor the properties”
Rather than spraying these nanoparticles on the surface by deposition techniques, they could be made by growing the nanoparticles directly from the backbone support, via exsolution. Exsolved nanoparticles have been recognized for their unique stability and reactivity. The atomic scale processes that control their formation were, however, not clear. Until now.
International team
Kyriakou and Tsampas teamed-up with researchers from the universities in Newcastle and Lyon to use the latest generation environmental transmission electron microscope to visualize the exsolution of individual nanoparticles and have detailed modelling of the process in parallel. Preliminary experiments with ex-situ analysis were conducted in order to define if the timescales of the exsolution process match with the ones of the operando microscopy. Tsampas, head of the research group Catalytic and Electrochemical Processes for Energy Applications: “This electron microscope provided the ultra-high spatial and temporal resolution to reveal the dynamics of atomic movement, particle formation and lattice reconstruction around the nanoparticles during exsolution.”
The detailed understanding of the exsolution process leads to new opportunities for controlling strain and reactivity at the nanoscale. Tsampas: “Our observations set new principles for the bottom up observation and tailoring of energy materials.”
Publication
In situ observation of nanoparticle exsolution from perovskite oxides: from Atomic scale mechanistic insight to nanostructure tailoring, D. Neagu, V. Kyriakou, I.-L. Roiban, M. Aouine, C. Tang, A. Caravaca, K. Kousi, I. Schreur-Piet, I.S. Metcalfe, P. Vernoux, M.C.M. van de Sanden, and M.N. Tsampas, ACS Nano, 21 October 2019 (link)
Science highlighted this publication in their issue of 15 November 2019, Watching metal nanoparticle exsolution (page 835).
Go to the News page.