A fresh look at photoelectrochemical cells may boost these devices' ability to convert sunlight into the clean energy carrier hydrogen. Researchers from DIFFER, Delft University of Technology and Twente University used carefully structured and measured photoelectrochemical cells in combination with software developed for urban design to explain why such cells only reach a fraction of their theoretical performance. It turns out that extending pillars on the cell surface can shadow each other, like skyscrapers in a busy city centre. The team published their research and a new design which may improve performance in the journal Advanced Functional Materials.
Hydrogen is a clean energy carrier that produces no greenhouse gas. That makes it an interesting option for the storage and transport of sustainable energy. At the moment, the cheapest way of producing hydrogen is through coal gassification or by steam reforming natural gas, with CO2 as a side product. To make hydrogen truly renewable in nature, scientists work on photoelectrochemical cells. These devices directly convert incoming energy from sunlight into the chemical process of splitting water into oxygen and hydrogen.
The trap of extra surface area
The bigger the area of a photoelectrochemical cell, the more reaction sites where hydrogen can be produced. To that end, researchers plant their cells with fields of microscopic pillars to increase the surface area. Such cells use a thin layer of tungsten oxide to redirect energy from sunlight into the water splitting reaction. A substrate of silicon boosts the process by converting sunlight more efficiently into the necessary charge carriers.
Improving photoelectrochemical cells often starts with tweaking material properties or by adding microstructures for an increased surface area. Improvements in those individual fields only partly translate into better performance, explains DIFFER group leader Anja Bieberle, the principal investigator in this project. "If you fill up a smooth photoelectrochemical cell with light sensitive nanowires, the surface may increase by a factor of over 50, but the cell performance only increases five times. How is that possible?"
Overview
To crack the riddle of diminishing returns, the team at DIFFER, Delft University of Technology and Twente University zoomed out to get a big picture view. They started by developing a series of photoelectrochemical cells with different substrates and light sensitive pillars on the surface, each cell with higher or lower pillars, packed closely together or further apart. Anja Bieberle: "These stepwise variations allowed us to know the exact differences between the individual cells. And again we saw that extra surface area did not result in the same amount of extra performance."
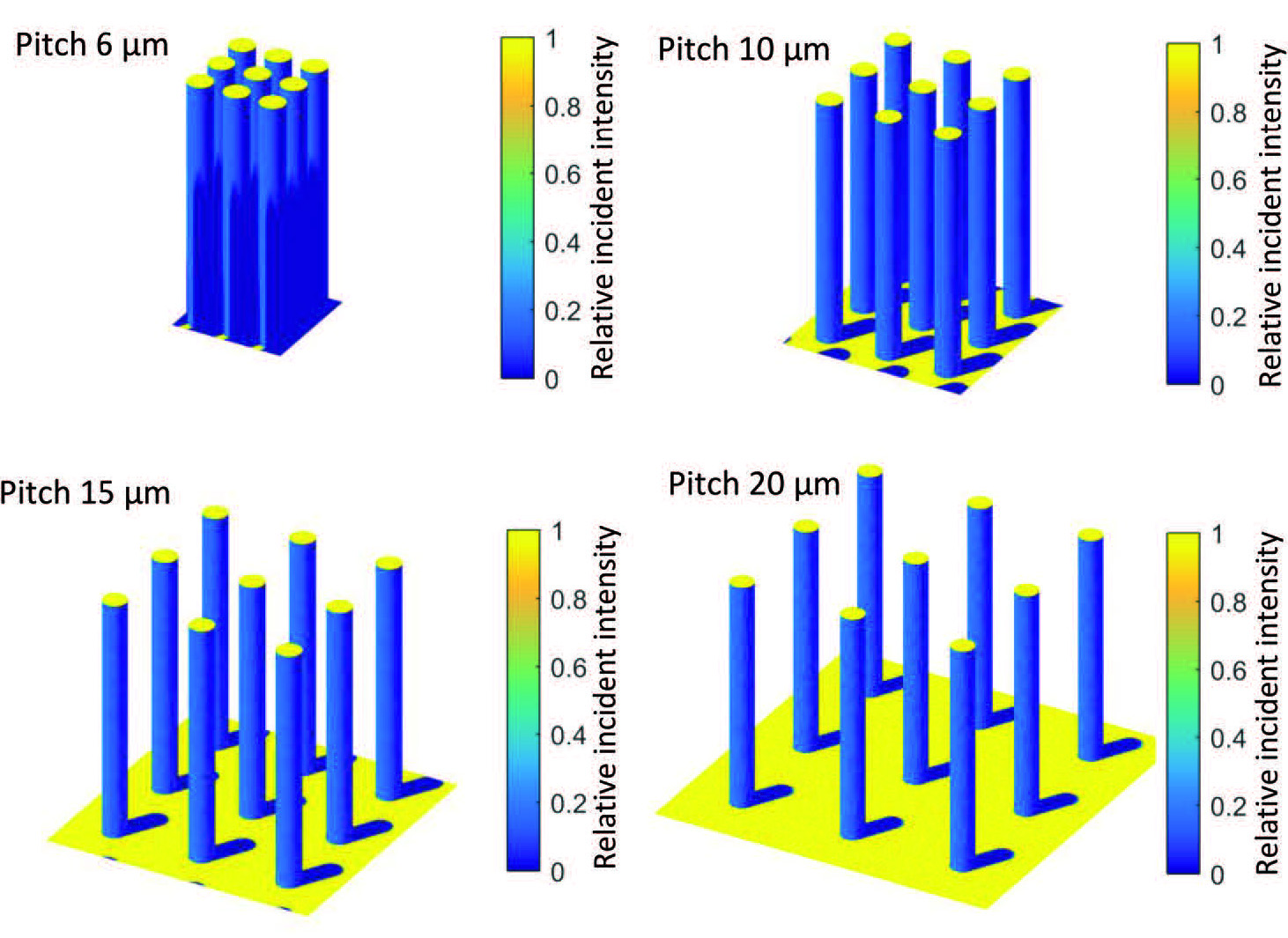
Software originally developed to determine the potential of solar energy in the urban environment delivered new insight into the problem. By simulating the microscopic pillars on their photoelectrochemical cells as if they were sky scrapers in a busy metropolis, the team discovered why extra pillars do not automatically produce extra hydrogen: if they are packed too closely together, the pillars shadow each other. No light on the surface means no energy for the water splitting reaction. In their publication, the scientists show that the choice of materials used can further influence the cell's performance.
Will this research allow us to squeeze more hydrogen out of our photoelectrochemical cells? In their publication, the team describe an alternative microscale design for such hydrogen cells. By swapping pillars for light sensitive cones, the protruding micro-towers can be placed closer together without shadowing each other.
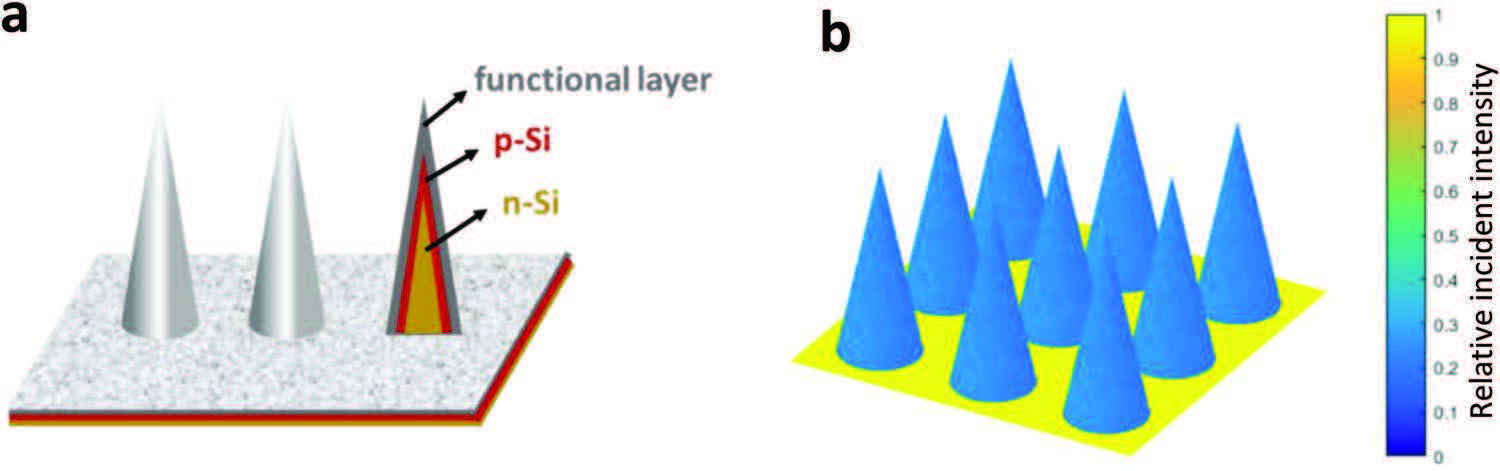
under illumination (light incident angle 1°).
An eye towards application
Anja Bieberle: "The next step in this research is to create such a photoelectrochemical cell filled with cones and check how well it performs." If that new design performs well, collaboration with industry for a scale-up is a logical next step. "We've made sure to use commercially relevant materials like silicon and tungsten oxide in our current work, so that it has direct bearing on real-world systems. The research is on fundamental properties, but always with an eye towards practical applications."
Publication
From Geometry to Activity: A Quantitative Analysis of WO3/Si Micropillar Arrays for Photoelectrochemical Water Splitting, Advanced Functional Materials (2020)
https://doi.org/10.1002/adfm.201909157
Go to the News page.