|
EXPERIMENTAL STUDIES
1. Development of complex photoelectrodes for efficient water splitting under visible light irradiation (PhD thesis Rochan Sinha) The aim of the project is to unravel the rate limiting steps at photo-electrochemical interfaces. For this, we fabricate and characterize well-defined, complex photo-electrodes which show stable and efficient photo-electrochemical activity. ‘Complex’ here pertains to both material composition as well as to the micro-/nanostructure. The project is integrated in the frame of combining experiments and modeling & simulations in order to identify the rate limiting steps at photo-electrochemical interfaces. |
|
|
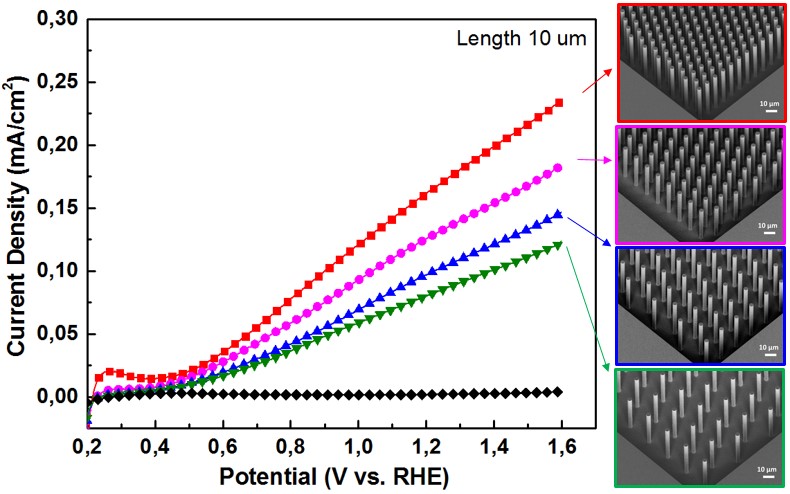
2. Innovative photoelectrode micro-/nanostructures and 3D structures (PhD thesis Yihui Zhao) Light harvesting and charge transportation properties are two crucial factors, which make micro-/nanostructure photoelectrodes essential to improve the efficiency of PEC water splitting. To design higher efficiency photoelectrode, it is important to discover the relation between the geometry and the PEC activity of photoelectrodes. In this study, we prepared well-defined micro- and nano- structured WO3/n-Si photoelectrodes and quantitatively analyze the relation between geometry and PEC activity. The aim of this research is to give a further understanding on the geometrical effects in PEC water splitting and provide a guidance for fabricating of new innovative 3D architectures. |
|
|
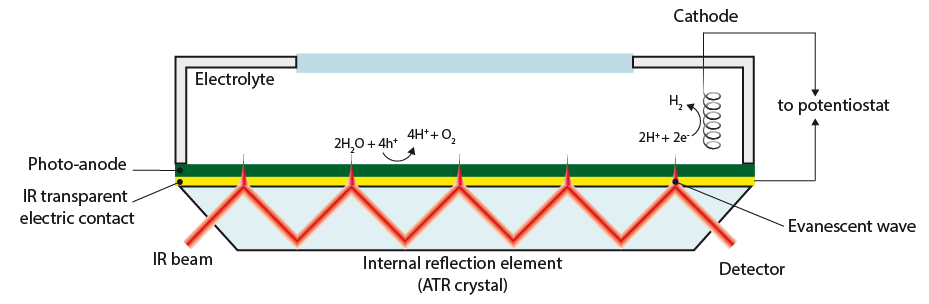
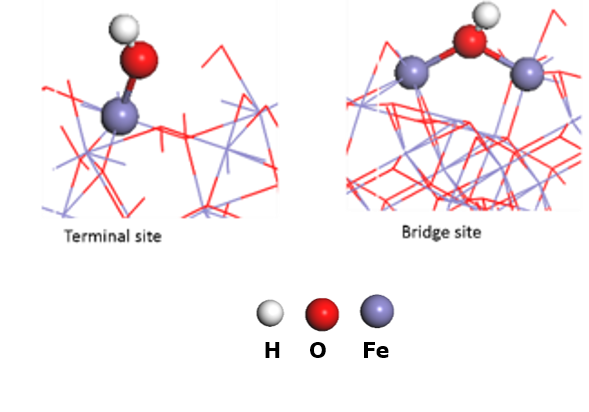
3. Detecting surface intermediates during water splitting (Post Doc Aafke Bronneberg) Which surface sites are involved in the water oxidation reaction? And which intermediates are formed at the surface of (photo)electrodes during water oxidation? Answers to these question will enable us to fabricate an electrode surface with a maximum number of these catalytic sites, thereby improving the overall water splitting efficiently. In addition, knowledge on surface intermediates is much needed input for modelling and simulation studies. In this project we aim to answer these questions using a novel method, based on attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), to detect the surface intermediates during actual water splitting conditions. (This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 708874)
|
|
|
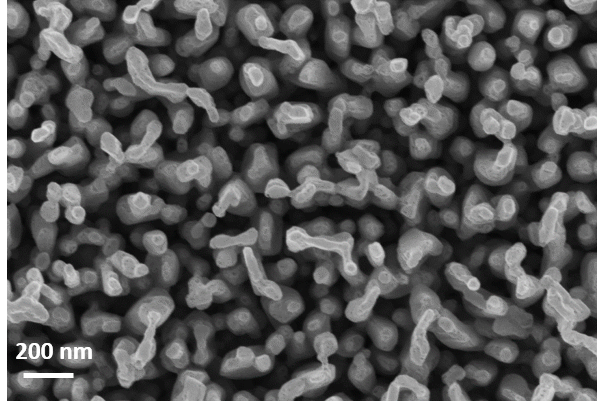
4. Plasma nanostructured photoelectrodes (contact: Anja Bieberle) In this project we study the feasibility of nanostructuring of thin metal and metal oxide films for possible application as photo-electrodes in solar fuel conversion. The formation of nanofuzz is a known phenomenon in fusion research due to plasma surface interaction caused by high ionic flux of the plasma. While the nanofuzz is not appreciated in fusion studies due to weakening of the walls, the large surface area of the materials is attractive for catalysis and electrochemistry. |
|
|
MODELING & SIMULATIONS
5. Density Functional Theory simulations of the Oxygen Evolution Reaction (Post doc Xueqing Zhang) Identifying the limiting of reaction steps in photo-electrochemical water splitting can help to maximize the performance and efficiency. In this project, we combine experiments and modeling & simulations. We simulate the oxygen evolution reaction (OER) and calculate the overpotentials and rate constants of each reaction step. Simulations are carried out on hematite surfaces with different orientations, surface terminations, and oxygen vacancy concentrations. The data will be used as input for the state-space modeling approach to simulate experimental photo-electrochemical data, such as impedance spectra. |
|
|
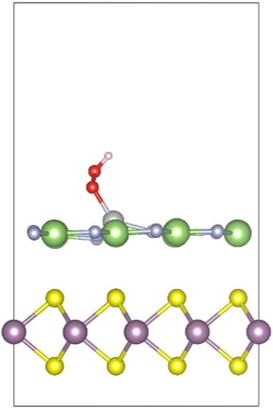
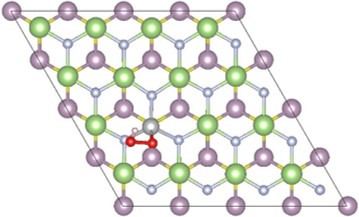
6. 2D Materials for Water Splitting (PhD thesis Qiuhua Liang) The reduction of the size is an efficient way to improve the photo-electrochemical performance of catalysts for the oxygen evolution reaction (OER). In recent years, two-dimensional (2D) layered materials and heterostructures have drawn great research efforts due to their high specific surface areas, abundant active sites and fast charge transfer rates. In this project, we mainly focus on the investigation of OER on 2D layered materials and heterostructures based on DFT calculation. The results can help to identify the overpotentials and rate constants of each reaction step, as well as pave the way for experimental research in the development of 2D materials for water splitting. |
  |
|
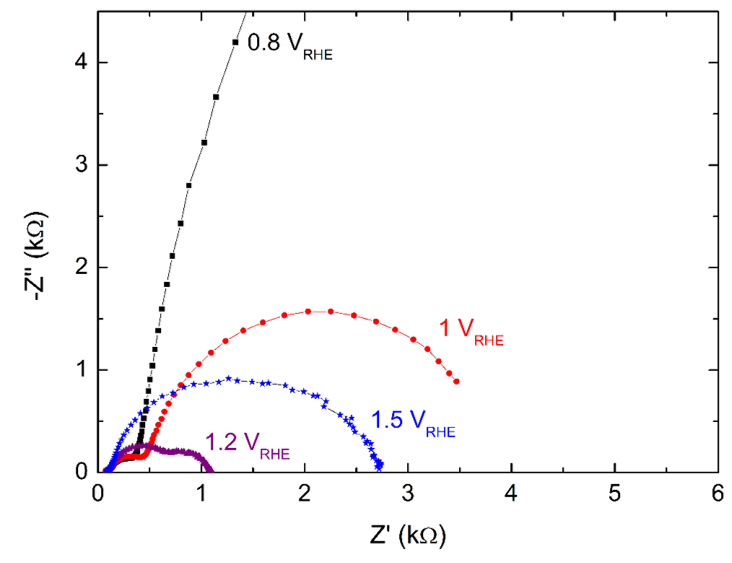
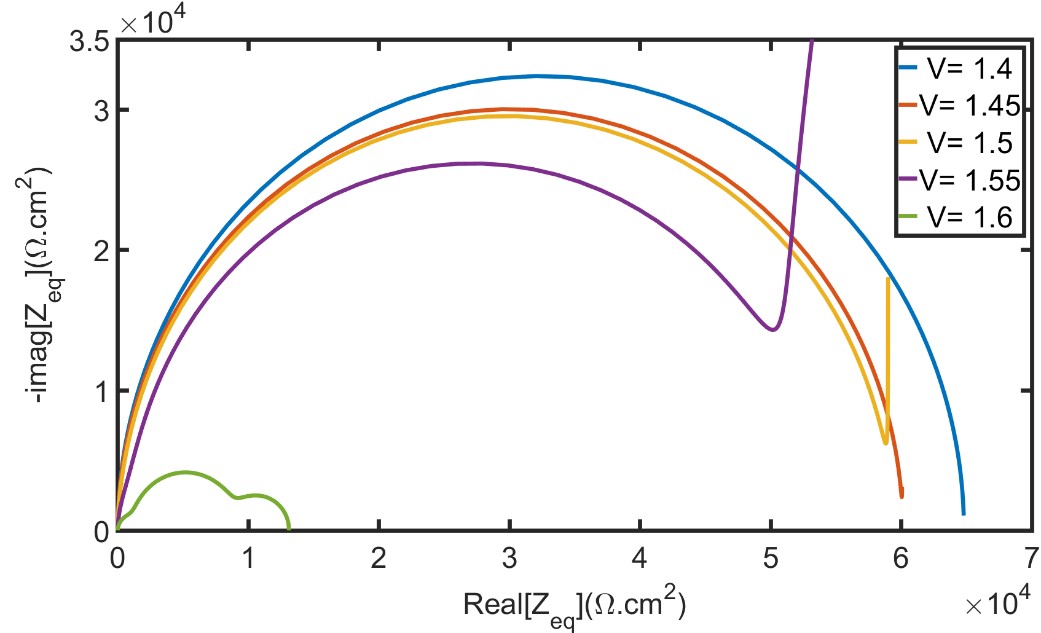
7. Simulating photo-electrochemical interface data by combining DFT and state-space modeling (PhD thesis Kiran George) The practical efficiency of photo electrochemical water splitting is found to be lower than theoretically predicted values. One of the reason for this is sluggish oxygen evolution reaction (OER) kinetics at the photoanode electrolyte interface. We use modeling and simulations to identify the rate limiting step in OER. It is proposed that OER proceeds as a four step reaction with four intermediate species. Any of these four reactions could be a rate determining reaction. The goal is to identify the reaction rate of each of these multiple steps by utilizing experimental data. For that, we have developed a state space model of the multi-step oxygen evolution reaction in Matlab/SIMULINK, with applied voltage as the input and current density as the output. From this model electrochemical data like the electrochemical impedance spectra and current density are simulated similar to experiments. The idea is to compare these simulated data from the model to experimental data and thereby identify the actual rates of each steps in the multi-step oxygen evolution reaction. The method uses density functional theory calculations (DFT) and Gerischer model of electron transfer for determining the intrinsic rate constants. The image shows impedance spectra at different voltages calculated using the developed model. |
|
|
|
Research
Electrochemical Materials and Interfaces