DIFFER is going to research a novel plasma-aided electrochemical reactor concept to cost-effectively convert small, common molecules into useful building blocks for the production of materials, chemicals and synthetic fuels. DIFFER researchers Floran Peeters and Richard van de Sanden scored an NWO ENW-XS project to fund their ground-breaking research project to replace the cathode of a conventional solid-oxide electrolyzer cell with a hollow cathode glow discharge fed by He/O2 gas.
Transforming the chemical industry to rely only on sustainable raw materials is a complicated task. How do we shape our material economy without relying on fossil fuels or scarce biological resources? Our answer: the use of sustainably generated electricity to convert common molecules such as water and carbon dioxide into chemical building blocks. And use a plasma as an efficient medium to channel electrical power into chemical energy!
DIFFER researcher Floran Peeters: “In this project, we will merge two developing electrochemical technologies: plasma conversion and solid oxide electrolysis. By generating a plasma on top of a solid oxide membrane, we hope to demonstrate oxygen atom conduction through the membrane without the need for expensive, complex and sensitive cathode materials needed to split oxygen molecules.” Group leader Richard van de Sanden further explains: “By essentially replacing the conventional cathode with a hollow cathode glow discharge, we expect to increase the flow of oxygen through the membrane, since the production of oxygen atoms from CO2 or H2O is much larger in a plasma than on the surface of a conventional cathode material.”
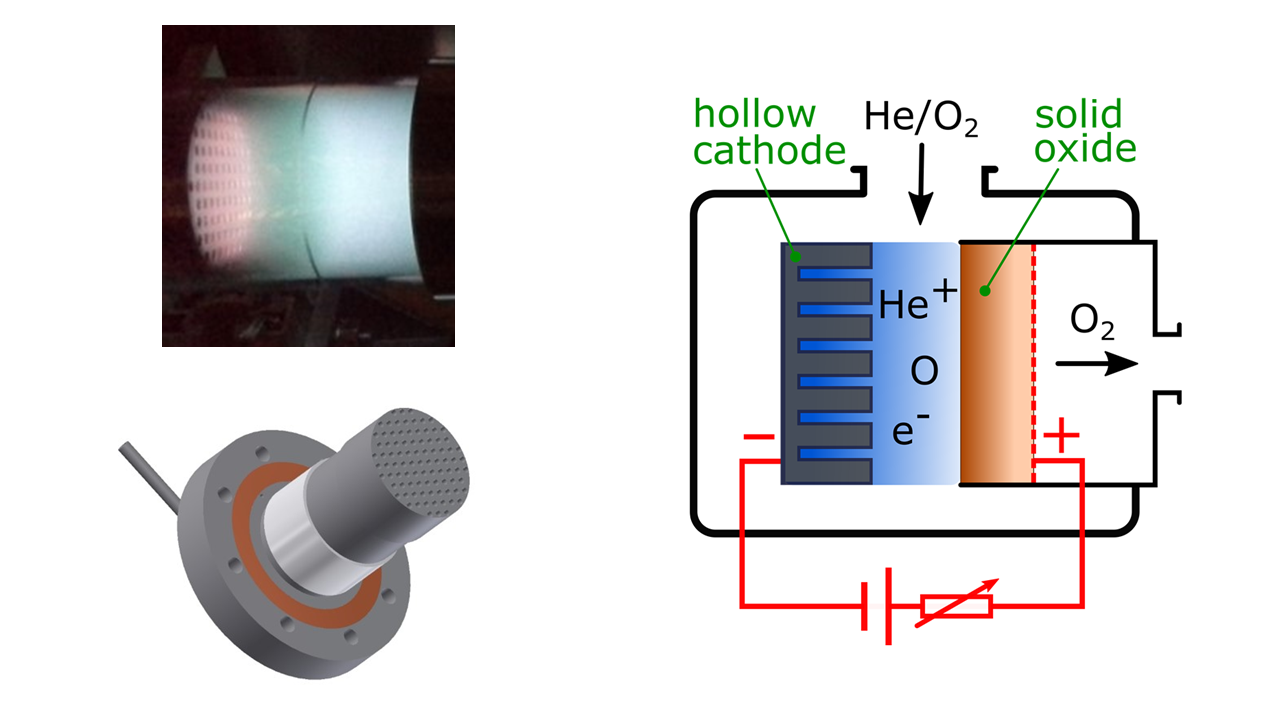
Floran Peeters: “To our knowledge, this will be the first time a merger between these two technologies is attempted. I am very motivated to investigate this novel concept which has both fundamental science questions as technological challenges.”
Experiments where a plasma and solid oxide electrolysis cell are operated side-by-side do exist, but not joined into a single electrochemical cell. If results proof positive, a solution to the major bottlenecks of both technologies presents itself: direct separation of conversion products in the plasma and enhanced durability, cost reduction and throughput for the solid oxide membrane. Richard van de Sanden agrees: “If the coupling proves successful, it provides the impetus for developing easily fabricated electrochemical reactors for sustainable, large-scale chemistry.”
More information on the NWO Open Competition Domain Science - Small-scale grants (NWO ENW-XS)
Go to the News page.