DIFFER researchers have demonstrated that coupling between electrolysis and plasmolysis leads to a novel route for CO2 conversion. The researchers are working in the frame of KEROGREEN, which is a European project on conversion of CO2 into green liquid fuels. The project ultimate goal is to build a container sized pilot plant at the Karlsruhe Institute of Technology (KIT) in Germany for the production of green aviation fuel.
Challenges with existing pathways
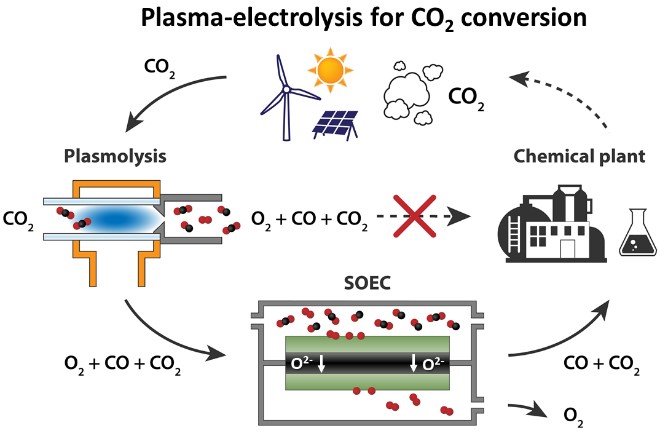
Liquid fuels for transportation and energy storage can be produced in a sustainable way from atmospheric CO2 driven by surplus electricity generated by wind turbines and solar panels. In this way the chemical building block for the fuels, the carbon monoxide (CO) molecule, can be produced in an unconventional way. Two such technologies for CO2 conversion are currently employed: high-temperature electrolysis and plasmolysis.
High-temperature electrolysis is characterized by a high yield and energy efficiency. This process results in the direct separation of the reaction products CO and oxygen (O2). However, it poses challenging requirements on electrode materials.
CO2 plasmolysis on the other hand, offers similar energy efficiencies, and no scarce electrode materials are required. However, the mixture of the reaction products cannot be used directly in a chemical synthesis plant. The CO produced remains mixed with O2 and residual CO2 and therefore an additional gas separation step is required.
Coupling of electrolysis and plasmolysis
DIFFER researchers have demonstrated that the coupling of electrolysis and plasmolysis leads to a renewable-electricity-driven route for CO production, overcoming the main bottleneck of CO2 plasmolysis. In the coupled process, the CO2 plasmolysis gas mixture is supplied to a high-temperature electrolyser to separate the product gases electrochemically using solid oxide electrolyte cells (SOEC). Oxygen is transferred to one side of the SOEC, where it is removed. The remaining CO and CO2 can be transported to a chemical plant for liquid fuel synthesis.
In a single pass, the coupled-process product contains 91% less O2 and 138% more CO compared with bare plasmolysis. "And what is more important: when the solid oxide electrolyte cells operate with the plasma mixture of CO, O2 and CO2 in the inlet, we didn't observe any electrode degradation in durability tests", says Michail Tsampas, leader of the DIFFER group Catalytic and Electrochemical Processes for Energy Applications. The synergy between plasmolysis and electrolysis opens up a novel route to efficient CO2 conversion into valuable building blocks for sustainable chemicals and fuels.
Publication
Arunkumar Pandiyan, Vasileios Kyriakou, Dragos Neagu, Stefan Welzel, Adelbert Goede, Mauritius C.M.van de Sanden, Mihalis N.Tsampas, CO2 conversion via coupled plasma-electrolysis process, Journal of CO2 Utilization, Volume 57, March 2022.
More information
The international KEROGREEN project
KEROGREEN at DIFFER
Go to the News page.